
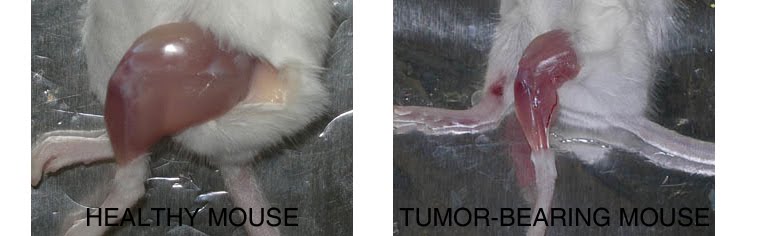
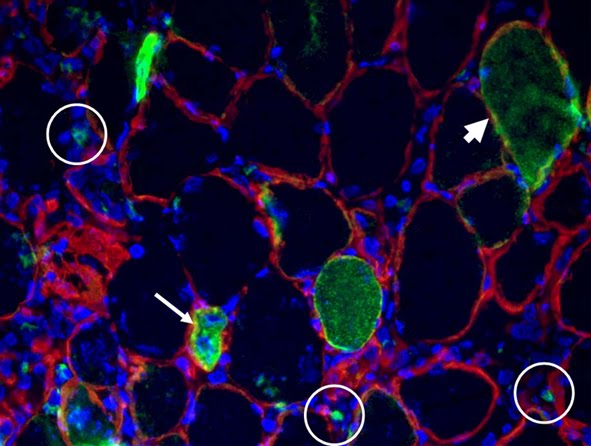
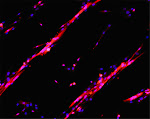
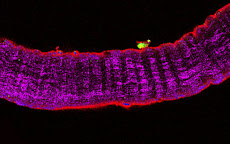
To celebrate a few recent events (the UPMC Emergence 2011 grant, the Mol Endocrinol paper) and to welcome a new student in the lab, we have tasted five Bordeaux 2006 wines, from different appellations characterized by marked nuances of their terroirs and specific grape assembly. Given that the different wineyards are only about 50 Km from each other, the differences were outstanding.
Results of the blind tasting (panel : laboratory members):
1st Château-Haut Maurac, Médoc Cru Bourgeois (60 % Cabernet sauvignon, 40 % Merlot)
2nd Château Musset Chevalier , Saint Emillon Grand cru (50 % Merlot noir / 45 % Cabernet-Franc / 5 % Cabernet-Sauvignon )
3rd Les Hauts du Tertre, Margaux (55 % Cabernet sauvignon, 20 % Merlot, 20 % Cabernet franc, 5 % Petit verdot)
4th Château Prieuré-les-Tours, Graves.
We liked the winner for its intense bouquet of red fruits and its full body, with mature tannins and a long lasting aftertaste. One more cru Borgeois showing the great quality/price ratio of this category. From the color to the marked tannins it expressed the Medoc pretty well. However, I preferred the Margaux of Les Hauts de Tertre, a second wine produced by Château du Tertre, for its elegance and its more floreal bouquet. Margaux came out in the good balance between tannins, acidity and alcoolic warmth. The superb roundness of the Libournais St Emillon and the acidity of the Graves (Alas! - in such a poor interpretation) came out as well, but nobody guessed the crus for all the wines.











No comments:
Post a Comment