RESEARCH INTERESTS: Cellular and molecular mechanisms of striated muscle physiopathology
Cancer cachexia
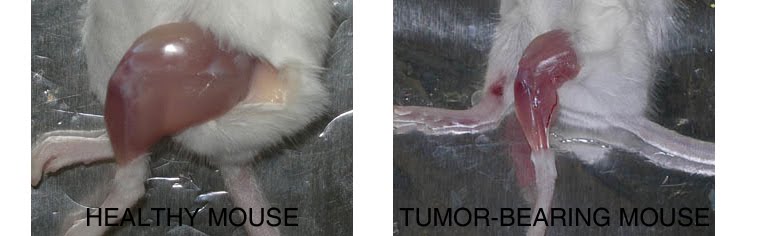
Compared to a control mouse (left) a tumor-bearing mouse (right) displays a dramatic muscle wasting. This loss of muscle mass is called cancer cachexia.
Exogenous gene expression in regenerating muscle
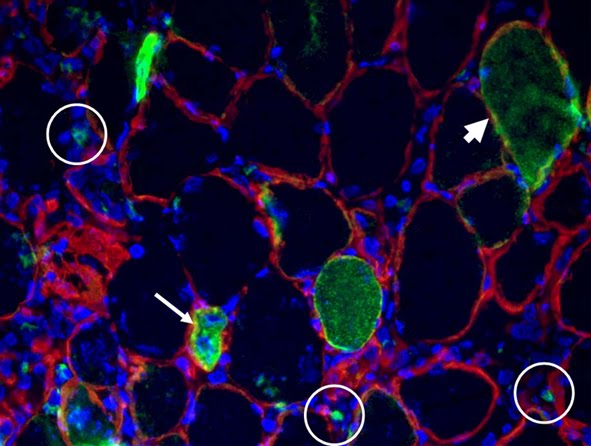
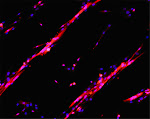
Depicted here is the over-expression of Green Fluorescent Protein (GFP, green; click on the image to access Tsien's Lab) in interstitial cells (circled), nascent myofibers (arrow) and adult fibers (arrowhead), in a regenerating Tibialis Anterior following focal injury. Laminin staining (red) highlights the basement membrane surrounding the skeletal muscle tissue, while nuclei are stained in blue. We do gene delivery by electroporation to study the regulation of muscle regeneration.
RESEARCH INTERESTS: Tissue engineering of skeletal muscle
Background and rationale.
Tissue engineering lies at the interface of regenerative medicine and developmental biology, and represent an innovative and multidisciplinary approach to build organs and tissues (Ingber and Levin, Development 2007). The skeletal muscle is a contractile tissue characterized by highly oriented bundles of giant syncytial cells (myofibers) and by mechanical resistance. Contractile, tissue-engineered skeletal muscle would be of significant benefit to patients with muscle deficits secondary to congenital anomalies, trauma, or surgery. Obvious limitations to this approach are the complexity of the musculature, composed of multiple tissues intimately intermingled and functionally interconnected, and the big dimensions of the majority of the muscles, which imply the involvement of an enormous amount of cells and rises problems of cell growth and survival (nutrition and oxygen delivery etc.). Two major approaches are followed to address these issues. Self-assembled skeletal muscle constructs are produced in vitro by delaminating sheets of cocultured myoblasts and fibroblasts, which results in contractile cylindrical “myooids.” Matrix-based approaches include placing cells into compacted lattices, seeding cells onto degradable polyglycolic acid sponges, seeding cells onto acellularized whole muscles, seeding cells into hydrogels, and seeding nonbiodegradable fiber sheets. Recently, decellularized matrix from cadaveric organs has been proven to be a good scaffold for cell repopulation to generate functional hearts in mice (Ott et al. Nature Medicine 2008).
I have obtained cultures of skeletal muscle cells on conductive surfaces, which is required to develop electronic device–muscle junctions for tissue engineering and medical applications1. I aim to exploit this system for either recording or stimulation of muscle cell biological activities, by exploiting the field effect transistor and capacitor potential of the conductive substratum-cell interface. Also, we are able to create patterned dispositions of molecules and cells on gold, which is important to mimic the highly oriented pattern myofibers show in vivo.
I have found that Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment2. Static magnetic field (SMF) interacts with mammal skeletal muscle; however, SMF effects on skeletal muscle cells are poorly investigated. 80 +/- mT SMF generated by a custom-made magnet promotes myogenic cell differentiation and hypertrophy in vitro. Finally, we have transplanted acellular scaffolds to study the in vivo response to this biomaterial3, which we want to exploit for tissue culture and regenerative medicine of skeletal muscle.
The specific aims of my current research are:
1) to increase and optimize the production and alignment of myogenic cells and myotubes in vitro;
2) to manipulate the niche of muscle stem cells aimed at ameliorating their regenerative capacity in vivo;
3) to develop muscle-electrical devices interactions. We plan to exploit the cell culture system on conductive substrates for either recording or stimulation of muscle cell biological activities, by exploiting the field effect transistor and capacitor potential of the conductive substratum-cell interface.
5) to produce pre-assembled, off-the-shelf skeletal muscle. We are seeding acellularized muscle scaffold with various cell types, with the goal to obtain functional muscle with vascular supply and nerves.
REFERENCES
1) Coletti D. et al., J Biomed Mat Res 2009; 91(2):370-377.
2) Coletti D. et al., Cytometry A. 2007;71(10):846-56.
3) Perniconi B. et al. Biomaterials, 2011 in press
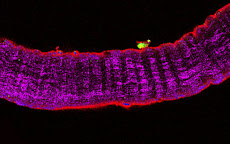
Cultures of myotubes on a conductive surface in a parallel orientation.
C2C12 cells cultured on gold, by mean of adhesion to 100 nm-wide stripes coated with anti Stem Cell antigen1 (Sca1) Ab. Nuclei (blue) and actin cytoskeleton (red) staining highlights the selective cells adhesion on the Ab-coated stripes and the formation of parallel multinucleated syncytia (myotubes).
12/05/2012
METHODS: 4 color IF for extra-cellular matrix and myosin isoforms
5/29/2012
METHODS: Visualisation of myosin isoforms by elecrophoresis and silver stain
5/21/2012
METHODS: Murine muscle dissection from the hinlimb
5/10/2012
EXPERIMENTAL MODELS: BALB/c substrains & running behavior
3/19/2012
Grip stenght test
3/05/2012
Indo-Italian Forum on Biomaterials and Tissue Engineering
2/28/2012
Candidate for ISAC councilor
2/19/2012
Quencing autofluorescence
THE NETWORK OF OUR COLLABORATORS 2017
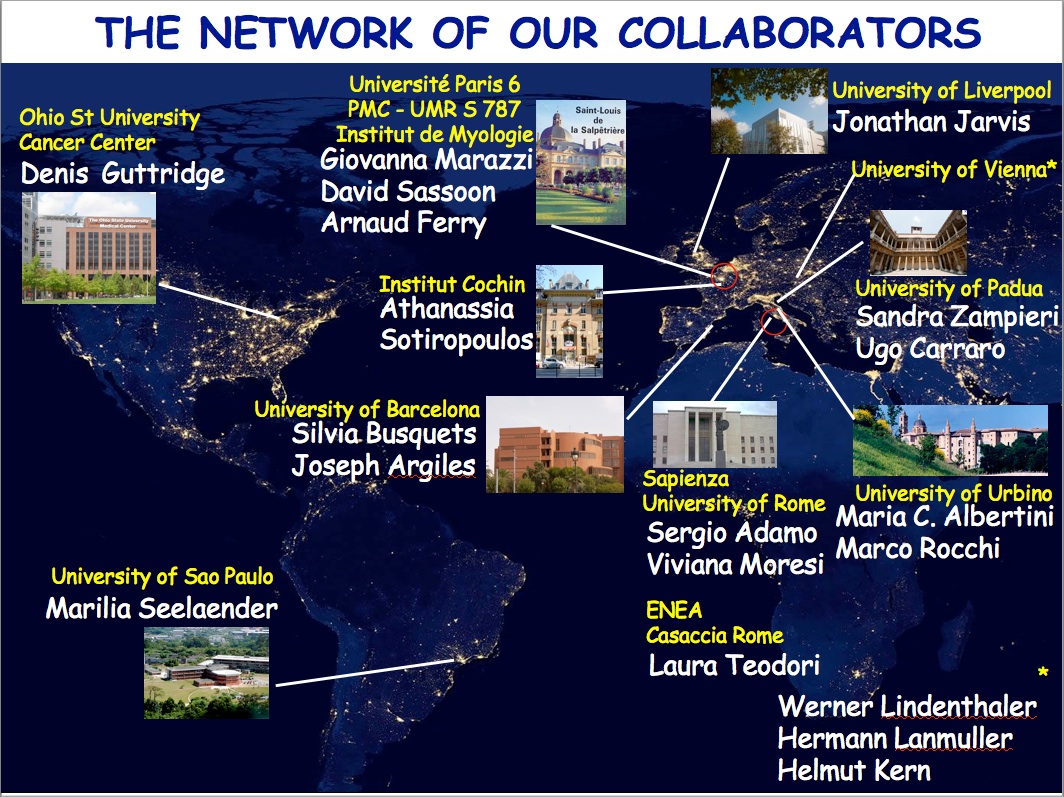
We collaborate with the Myology Group and the Cochin Hospital in Paris for stem cell studies and SRF, with the Cancer Centre at Ohio State University, Columbus for studies on the mechanisms underlying cachexia, with the Neurorehabilitation Unit at University of Pisa for clinical studies, with Pharmacology and Bioinformatics at the University of Urbino for advanced statistical analyses, with the Anatomy Section at the University of Perugia and with GYN/OB at the University of Western Piedmont for studies related to circulating factors and myogenic cell responses in cachexia, with the Biotech-Med Unit at ENEA, Chemistry in Rome and Anatomy in palermo for tissue engineering applications. Functional studies are carried out in our Departement in Rome in collaboration with Musaro's laboratory.