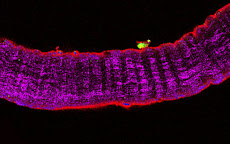
RESEARCH INTERESTS: Cellular and molecular mechanisms of striated muscle physiopathology
Cancer cachexia
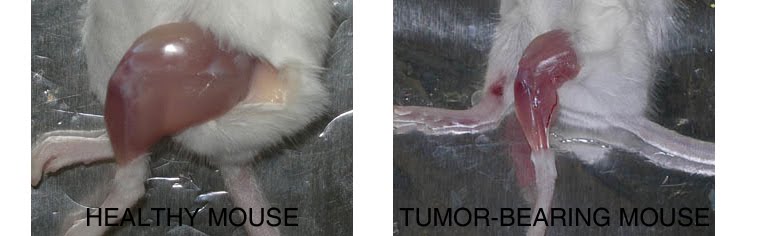
Compared to a control mouse (left) a tumor-bearing mouse (right) displays a dramatic muscle wasting. This loss of muscle mass is called cancer cachexia.
Exogenous gene expression in regenerating muscle
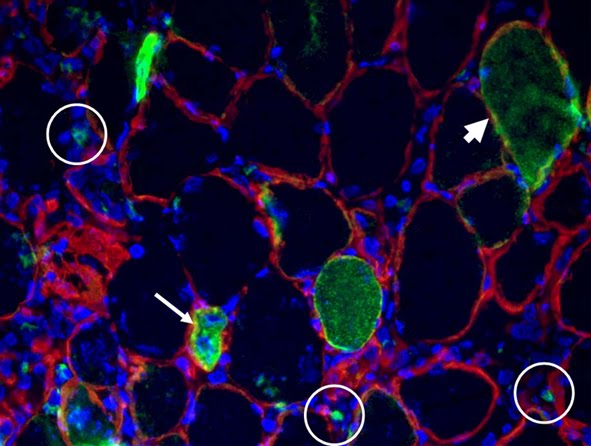
Depicted here is the over-expression of Green Fluorescent Protein (GFP, green; click on the image to access Tsien's Lab) in interstitial cells (circled), nascent myofibers (arrow) and adult fibers (arrowhead), in a regenerating Tibialis Anterior following focal injury. Laminin staining (red) highlights the basement membrane surrounding the skeletal muscle tissue, while nuclei are stained in blue. We do gene delivery by electroporation to study the regulation of muscle regeneration.
RESEARCH INTERESTS: Tissue engineering of skeletal muscle
Background and rationale.
Tissue engineering lies at the interface of regenerative medicine and developmental biology, and represent an innovative and multidisciplinary approach to build organs and tissues (Ingber and Levin, Development 2007). The skeletal muscle is a contractile tissue characterized by highly oriented bundles of giant syncytial cells (myofibers) and by mechanical resistance. Contractile, tissue-engineered skeletal muscle would be of significant benefit to patients with muscle deficits secondary to congenital anomalies, trauma, or surgery. Obvious limitations to this approach are the complexity of the musculature, composed of multiple tissues intimately intermingled and functionally interconnected, and the big dimensions of the majority of the muscles, which imply the involvement of an enormous amount of cells and rises problems of cell growth and survival (nutrition and oxygen delivery etc.). Two major approaches are followed to address these issues. Self-assembled skeletal muscle constructs are produced in vitro by delaminating sheets of cocultured myoblasts and fibroblasts, which results in contractile cylindrical “myooids.” Matrix-based approaches include placing cells into compacted lattices, seeding cells onto degradable polyglycolic acid sponges, seeding cells onto acellularized whole muscles, seeding cells into hydrogels, and seeding nonbiodegradable fiber sheets. Recently, decellularized matrix from cadaveric organs has been proven to be a good scaffold for cell repopulation to generate functional hearts in mice (Ott et al. Nature Medicine 2008).
I have obtained cultures of skeletal muscle cells on conductive surfaces, which is required to develop electronic device–muscle junctions for tissue engineering and medical applications1. I aim to exploit this system for either recording or stimulation of muscle cell biological activities, by exploiting the field effect transistor and capacitor potential of the conductive substratum-cell interface. Also, we are able to create patterned dispositions of molecules and cells on gold, which is important to mimic the highly oriented pattern myofibers show in vivo.
I have found that Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment2. Static magnetic field (SMF) interacts with mammal skeletal muscle; however, SMF effects on skeletal muscle cells are poorly investigated. 80 +/- mT SMF generated by a custom-made magnet promotes myogenic cell differentiation and hypertrophy in vitro. Finally, we have transplanted acellular scaffolds to study the in vivo response to this biomaterial3, which we want to exploit for tissue culture and regenerative medicine of skeletal muscle.
The specific aims of my current research are:
1) to increase and optimize the production and alignment of myogenic cells and myotubes in vitro;
2) to manipulate the niche of muscle stem cells aimed at ameliorating their regenerative capacity in vivo;
3) to develop muscle-electrical devices interactions. We plan to exploit the cell culture system on conductive substrates for either recording or stimulation of muscle cell biological activities, by exploiting the field effect transistor and capacitor potential of the conductive substratum-cell interface.
5) to produce pre-assembled, off-the-shelf skeletal muscle. We are seeding acellularized muscle scaffold with various cell types, with the goal to obtain functional muscle with vascular supply and nerves.
REFERENCES
1) Coletti D. et al., J Biomed Mat Res 2009; 91(2):370-377.
2) Coletti D. et al., Cytometry A. 2007;71(10):846-56.
3) Perniconi B. et al. Biomaterials, 2011 in press
Cultures of myotubes on a conductive surface in a parallel orientation.
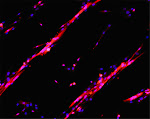
C2C12 cells cultured on gold, by mean of adhesion to 100 nm-wide stripes coated with anti Stem Cell antigen1 (Sca1) Ab. Nuclei (blue) and actin cytoskeleton (red) staining highlights the selective cells adhesion on the Ab-coated stripes and the formation of parallel multinucleated syncytia (myotubes).
11/29/2010
ARTICLES: The Problem of Subjective/Objective Genitive in Matters of Heart
Comment on:
Cell, 20 August 2010, Volume 142, Issue 4, 531 - 543
doi:10.1016/j.cell.2010.07.011
Article
Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival
Xiaolan Zhou, Jin Lin Wang, John Lu, Yanping Song, Keith S. Kwak, Qingsheng Jiao, Robert Rosenfeld, Qing Chen, Thomas Boone, W. Scott Simonet, David L. Lacey, Alfred L. Goldberg, and H.Q. Han
by:
Dario Coletti
Barbara Perniconi, Sergio Adamo, Zhenlin Li, Denise Paulin, Mathias Mericskay
19 novembre 2010
10:21:04 HNEC
Affiliation:University Pierre et Marie Curie Paris 6, France & Sapienza University of Rome, Italy
The Problem of Subjective/Objective Genitive in Matters of Heart
Zhou et al. uncovered a previously unappreciated loss of heart mass in cachectic mice for which, from our point of view, they correctly used the expression “atrophy of the heart”. This study is likely to generate a new line of research, which is distinct from cardiac cachexia, i.e. the atrophy of the skeletal muscle induced by cardiac pathologies. We urge to clarify the terminology to describe these phenomenons, since we foresee the risk of misleading use of related expressions, e.g. cardiac cachexia and cardiac atrophy, sounding alike but very different de facto one from the other. In particular, “cardiac cachexia” poses a problem of ambiguity, thus its use might be abandoned.
The old problem of the subjective/objective genitive case.
Does amor patris (father's love) mean that the father (pater) loves his children (subjective genitive) or that the children love their father (objective genitive)? The father can be either the subject or the object of the action of loving. There is no difference in form between the subjective and the objective genitive. Only context can make a final determination. In addition, English does not typically mark nouns for a genitive case morphologically. Rather, it uses the Saxon genitive “ 's” for people or the preposition “of” like in “Molecular Biology of the Cell”. Biology of the cell can also be referred to as “Cell biology” by using the noun as adjective.
What is cardiac cachexia, then?
About 200 papers to date referred to cardiac cachexia as a syndrome of skeletal muscle wasting associated to a specific pathology, i.e. Chronic Heart Failure (CHF). Cardiac cachexia is not meant as cachexia of the heart, i.e. cardiac atrophy. However, the existence of a cardiac component of cardiac cachexia has also been reported (Florea et al., 2002). Several papers demonstrate the growing use of “muscle cachexia” referred to as skeletal muscle wasting associated to a chronic disease, including CHF (Pajak et al., 2008). Following the recent discoveries of cardiac wasting in cachexia of both cardiac and non-cardiac origin (Florea et al., 2002, Zhou et al., 2010), it appears that the heart can be both the trigger and the target of cachexia. We are concerned that, by analogy to muscle cachexia, the use of “cardiac cachexia” may arise in a double, subjective/objective sense.
Different names for cardiac muscle wasting: PROs and CONs.
The following are alternative ways to refer to the phenomenon of cardiac muscle wasting.
1) Atrophy of the heart. This expression is unambiguous, even though relatively long. It is very general and implies the need to clarify the context in which the atrophy arises, e.g. cancer-induced atrophy of the heart to specify that the latter is triggered by cancer.
2) Heart atrophy, which is formally ambiguous in terms of subjective/objective genitive.
3) Cardiac atrophy, which has the same risk and sounds dangerously similar to cardiac cachexia.
4) Cachectic heart, a more holistic expression not entirely described to date.
5) Cardiac wasting, which has a nuance toward pathology and appears as a specific, easily discernible expression. Therefore, we are in its favor.
Highlighting the atrophy of the heart in the definition of cachexia.
The current consensus definition of cachexia, “Cachexia is a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle [...]” (Evans et al., 2008), does not explicitly refers to the loss of cardiac muscle. We think opportune to refer to both skeletal and cardiac musculature when reporting about muscle wasting in cachexia.
References
Evans, W.J. et al. (2008). Clinical nutrition (Edinburgh, Scotland) 27, 793-799
Florea, V.G. et al. (2002). American heart journal 144, 45-50
Pajak, B. et al. (2008). J Physiol Pharmacol 59 Suppl 9, 251-264
Published online on 11/19/2010
http://www.cell.com/comments/S0092-8674(10)00780-4
THE NETWORK OF OUR COLLABORATORS 2017

We collaborate with the Myology Group and the Cochin Hospital in Paris for stem cell studies and SRF, with the Cancer Centre at Ohio State University, Columbus for studies on the mechanisms underlying cachexia, with the Neurorehabilitation Unit at University of Pisa for clinical studies, with Pharmacology and Bioinformatics at the University of Urbino for advanced statistical analyses, with the Anatomy Section at the University of Perugia and with GYN/OB at the University of Western Piedmont for studies related to circulating factors and myogenic cell responses in cachexia, with the Biotech-Med Unit at ENEA, Chemistry in Rome and Anatomy in palermo for tissue engineering applications. Functional studies are carried out in our Departement in Rome in collaboration with Musaro's laboratory.